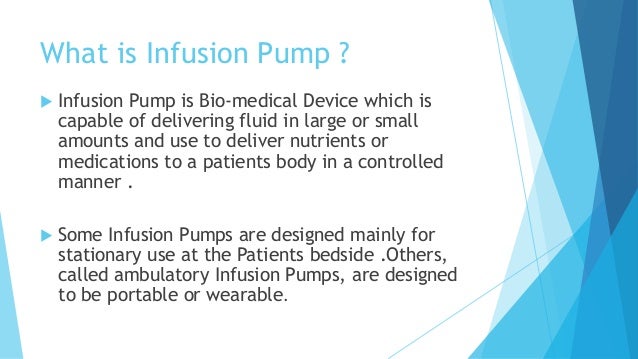
Infusion Pump Life Cycle
infusion life pump wallpaperThe Food and Drug Administration FDA recently issued the guidance document entitled Infusion Pumps Total Product Life Cycle - Guidance for Industry and FDA Staff which aimed to assist infusion pump manufacturers validate safety claims in 510k premarket notification submissions. Life cycle costs also see LCC are the total costs incurred throughout the service life of a pump system.
Https Www Draeger Com Products Content V500 V300 Vn500 Battery Concept Pi 9103811 En Pdf
Sterile hypodermic syringes for single use - Part 2.
Infusion pump life cycle. AIVs sales team provides the necessary expertise to help your facility choose the right equipment for your needs. However even with modern insulin pumps errors of insulin infusion can occur due to pump failure insulin infusion set IIS blockage infusion site problems insulin stability issues user error or a. Syringes for use with power-driven syringe pumps 6-273 ISO 23908 First edition 2011-06-11 Sharps injury protection - Requirements and test methods - Sharps protection features for single-use hypodermic needles introducers for catheters and needles used for blood sampling.
Continuous infusion usually consists of small pulses of infusion usually between 500 nanoliters and 10 milliliters depending on the pumps design with the rate of these pulses depending on the programmed infusion speed. Insulin pump therapy also known as continuous subcutaneous insulin infusion CSII is an important and evolving form of insulin delivery which is mainly used for people with type 1 diabetes. FDA believes that these recommendations will help mitigate risks and reduce future risks associated with infusion pumps.
Clinicians can attach up to four infusion modules allowing four independent infusions on a single BD Alaris PC unit. Total Pump Management Life Cycle November 10 2013. In this case the recommendations are intended to improve the quality of infusion pumps in order to reduce the number of recalls and infusion pump Medical Device Reports MDRs.
The metrics also reduce the number of new pumps needed and indicate which pumps can be set for daily maintenance cycles while they are unused. We are committed to leverage our distinctive competencies in Life Cycle Management to be your first choice for FDAISO compliant infusion devices contract manufacturing and QARA consulting services. David Lim PhD RAC ASQ-CQA LinkedIn The US Food and Drug Administration FDA issued a final guidance dated 2014-12-02 entitled Infusion Pumps Total Product Life Cycle The guidance provides assistance to industry in preparing premarket submissions for infusion pumps and to identify device features that manufacturers should address throughout the total product life cycle.
Guidance for Industry and FDA Staff to the Office of the Center Director Guidance and Policy Development Center for Devices and Radiological Health Food and Drug Administration 10903 New Hampshire Ave Bldg. Submit written requests for a single hard copy of the guidance document entitled Infusion Pumps Total Product Life Cycle. The draft of this document was issued on April 23 2010.
WellSpan Health completed a capital budget evaluation of smart infusion device technology in 2008 when our current infusion devices were reaching the end-of-life phase and the manufacturer indicated they would no longer be supported because the lack of smart pump functionality made them obsolete. The BD Alaris pump module is a large volume infusion pump that continuously or intermittently delivers fluids medications blood and blood products to adult pediatric or neonatal patients. We offer sales and support of quality refurbished.
Guidance for Industry and FDA Staff. Assists in preparing premarket submissions for infusion pumps and to identify device features that should be addressed throughout the total product life cycle. The life cycle costs associated with the operation of a pump or pump system are determined by calculating the annual costs of operation plus the interest and depreciation for the non-current assets like.
They are used to compare the economic efficiency of various technical designs. Entire product life cycle. Guidance on the Content of Premarket Notification 510k Submissions for External Infusion Pumps 03011993 Infusion Pumps Total Product Life Cycle - Guidance for Industry and FDA Staff 12022014.
InfuLife Infusion Pump Pain Management Kits. Guidance for Industry and FDA Staff to the Office of the Center Director Guidance and Policy Development Center for Devices and Radiological Health Food and Drug Administration 10903 New Hampshire Ave Bldg. The LCC equation is determined in accordance with the guidelines of EUROPUMP and the Hydraulic Institute.
Knowing which infusion pumps are at work and at what times allows the hospital to move 100 pumps from over utilized to under utilized locations while renewing their pump fleet. Infusion Pumps Total Product Life Cycle. Medical Equipment Life Expectancy List EQUIPMENT LIFE EXPECTANCY IN YEARS Absorptiometer Dual Photon X-ray 8.
US FED STD 209E class 100000 Cleanroom Manufacturing. We can extend the life of your infusion pumps and save you money by limiting your capital expenditure purchases. Infusion Pumps FDA Total Product Life Cycle TPLC Guidance.
Submit written requests for a single hard copy of the guidance document entitled Infusion Pumps Total Product Life Cycle. The user interface of pumps usually requests details on the type of infusion from the technician or nurse that sets them up. ISO certified class 8.
Http Brand Med Com Upload News 180711 Operationmanualofbd5000syringepump Pdf
 Infusion Pumps Supplies Medicare Gov Infused Health Plan Pumps
Infusion Pumps Supplies Medicare Gov Infused Health Plan Pumps
Jmis Journal Of Multimedia Information System
 Cadd Solis Vip Ambulatory Infusion Pump Infusion Smiths Medical
Cadd Solis Vip Ambulatory Infusion Pump Infusion Smiths Medical
Https Assistinternational Org Wp Content Uploads 2020 05 Htm Session 6 Infusion Syringe Pumps V5 1 Pdf
Jhf A Hazard Analysis Of Class I Recalls Of Infusion Pumps Gao Jmir Human Factors
 Infusion Pumps General Purpose Ecri Institute
Infusion Pumps General Purpose Ecri Institute
 Safety Assurance Cases A Way To Shorten Painful Discussions With Auditors
Safety Assurance Cases A Way To Shorten Painful Discussions With Auditors
 System Architecture Of Generic Insulin Infusion Pump Download Scientific Diagram
System Architecture Of Generic Insulin Infusion Pump Download Scientific Diagram
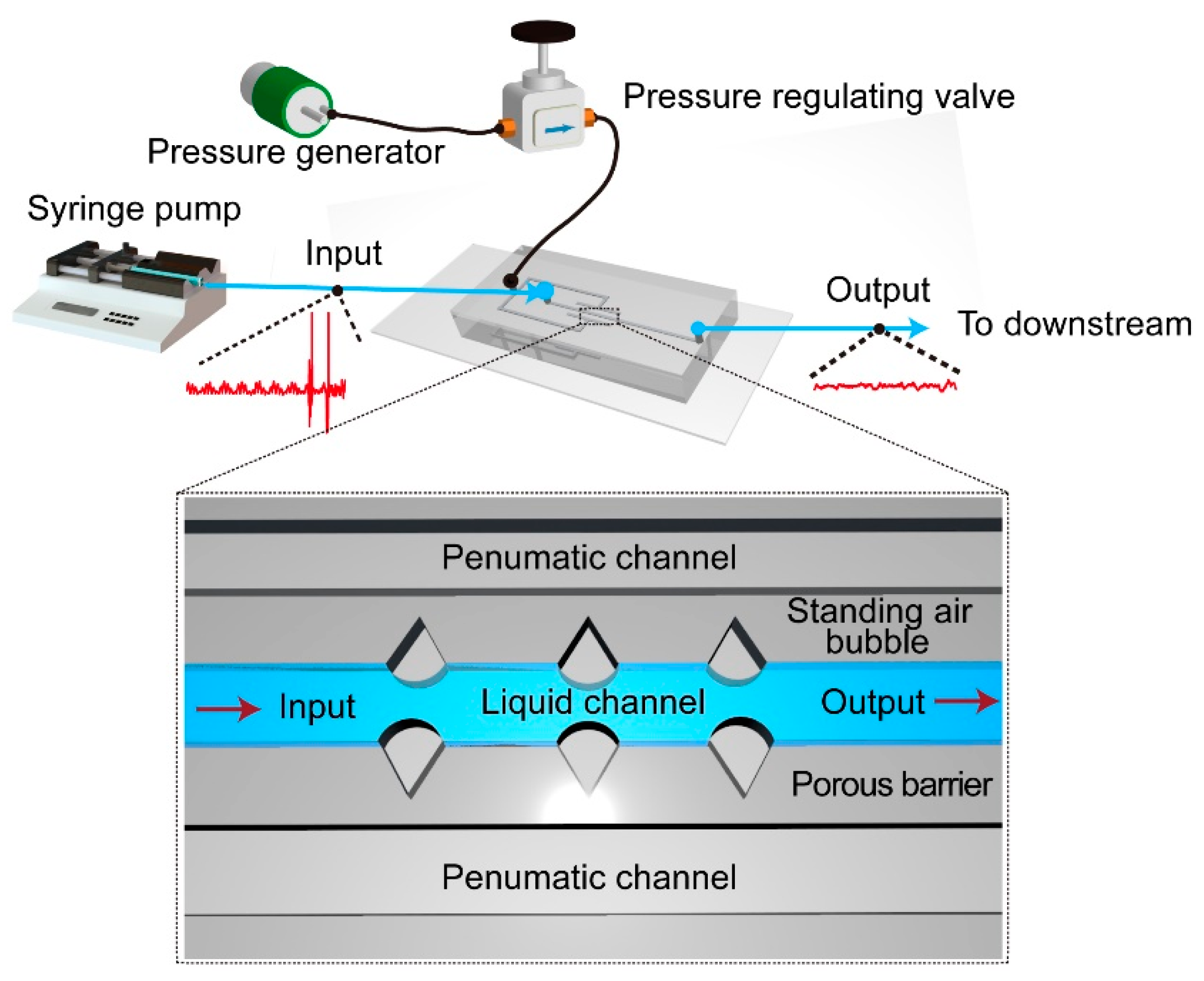 Micromachines Free Full Text Standing Air Bubble Based Micro Hydraulic Capacitors For Flow Stabilization In Syringe Pump Driven Systems Html
Micromachines Free Full Text Standing Air Bubble Based Micro Hydraulic Capacitors For Flow Stabilization In Syringe Pump Driven Systems Html
 Graseby 3200 Infusion Pump Service Manual Internetmed
Graseby 3200 Infusion Pump Service Manual Internetmed
Https Ibisuva Nl Assets Publicaties Quandaries Qq 2009 4 Infusion Pumps Pdf
 Any Kind Of Training Or Documentation Requirement Please Contact Us At 9811634856 Or Visit Www Iqmsglobal Com For Further Informa In 2020 Life Cycles Iso 13485 Medical
Any Kind Of Training Or Documentation Requirement Please Contact Us At 9811634856 Or Visit Www Iqmsglobal Com For Further Informa In 2020 Life Cycles Iso 13485 Medical
 Smart Infusion Pumps By Your Hospital Bed Health Tech Weekly Critical Care Nursing Icu Health Tech
Smart Infusion Pumps By Your Hospital Bed Health Tech Weekly Critical Care Nursing Icu Health Tech
 Pdf August 2005 Product Comparison Infusion Pumps General Purpose Umdns Information Adi Widjaya Academia Edu
Pdf August 2005 Product Comparison Infusion Pumps General Purpose Umdns Information Adi Widjaya Academia Edu
 Hospira Plum A Infusion Pump Medical Equipment Diamedical Usa
Hospira Plum A Infusion Pump Medical Equipment Diamedical Usa
 Infusion Pump Medical Equipment Medical Medical Device
Infusion Pump Medical Equipment Medical Medical Device
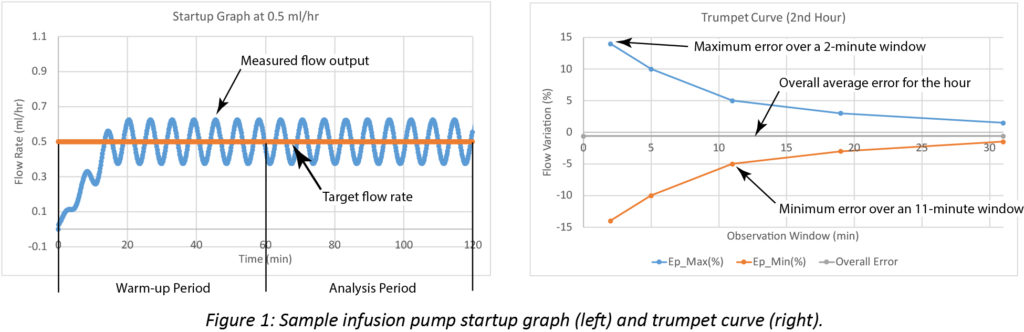 Infusion Pump Performance Flow Accuracy And Continuity Often Don T Add Up Patient Safety Quality Healthcare
Infusion Pump Performance Flow Accuracy And Continuity Often Don T Add Up Patient Safety Quality Healthcare
 Graseby Omnifuse Infusion Pump Operaters Manual Internetmed
Graseby Omnifuse Infusion Pump Operaters Manual Internetmed